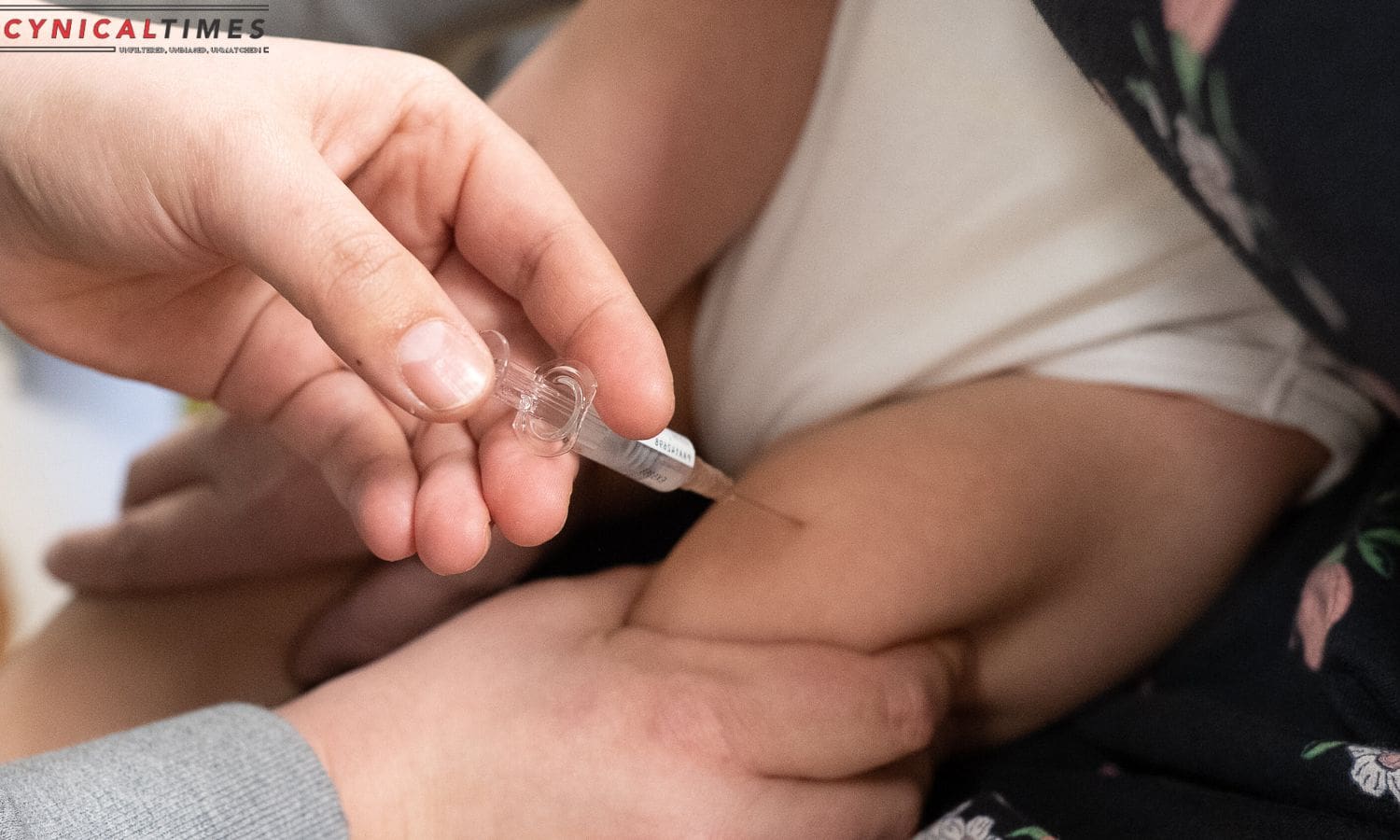
RSV Vaccine Shortage : In a new challenge for parents seeking to safeguard their infants from respiratory syncytial virus (RSV) this winter, the manufacturer has halted the acceptance of orders for some doses due to an overwhelming surge in demand.
Given the limited supply, the US Centers for Disease Control and Prevention has advised that doctors prioritize scarce doses of the novel therapy, nirsevimab, marketed as Beyfortus, for the most vulnerable infants. This includes those under six months of age and those with underlying health conditions increasing their risk of severe RSV.
Furthermore, the CDC recommends that doctors discontinue the use of Beyfortus for babies aged 8 to 19 months who are eligible for an older protective therapy called palivizumab or Synagis. Synagis is administered to children at high risk of severe RSV due to significant lung or heart conditions. Unlike Beyfortus, which provides six months of protection with a single dose, Synagis requires monthly administration throughout the RSV season.

Dr. Buddy Creech, a pediatrician at Vanderbilt and president of the Pediatric Infectious Disease Society, expressed the frustration of being unable to protect as many children as hoped, despite the commendable efforts to safeguard some.
These new guidelines imply that healthy infants over six months old may miss out on the innovative protection offered by Beyfortus.
Research from the previous RSV season revealed that 81% of infants requiring intensive care had no underlying medical conditions, making Beyfortus a crucial intervention. The therapy, approved by the US Food and Drug Administration, had garnered strong recommendations from the CDC for babies under 8 months entering their first RSV season, as well as for specific high-risk toddlers up to the age of 2.
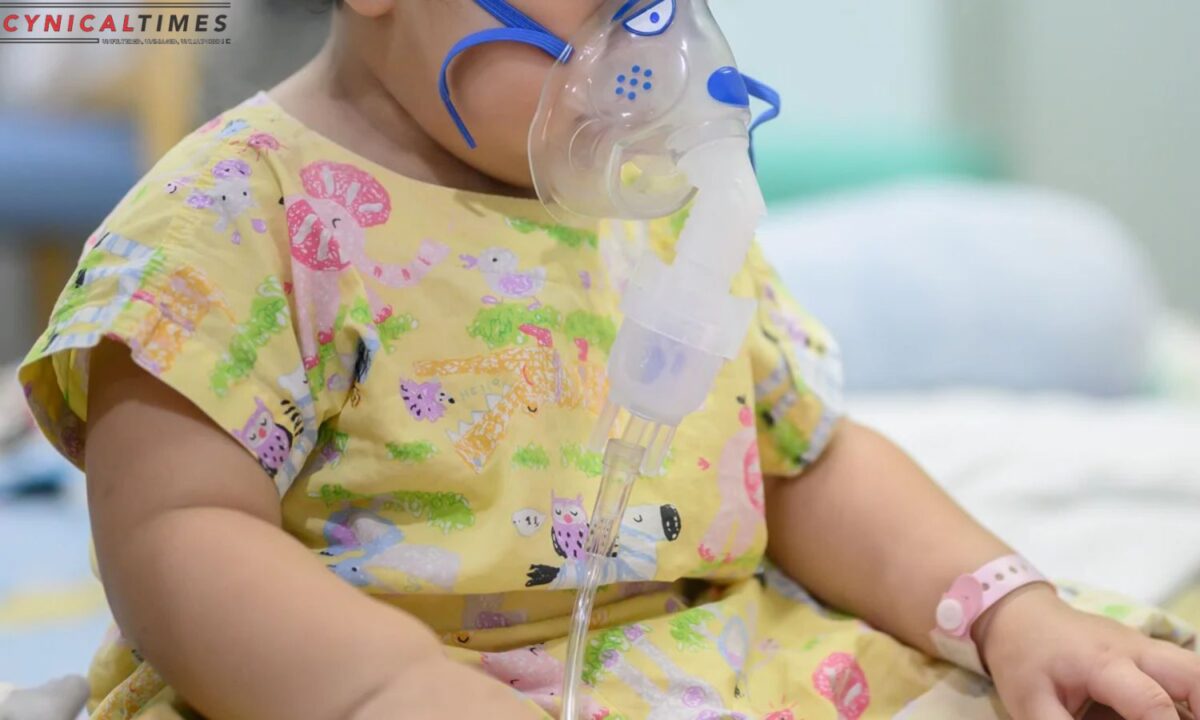
RSV targets the lower lungs, leading to the accumulation of mucus, which can obstruct the tiny airways of infants, making breathing and feeding difficult. It’s the primary cause of hospitalization among infants under one year of age.
Pediatricians, eager to protect their youngest patients, found themselves contemplating the costs and reimbursement complexities associated with Beyfortus. The new shots retail at around $500 per dose, making it a potential financial challenge for parents to access.

Also Read : Pink Tax in Healthcare: Women Financial Struggle for Equal Access
One manufacturer, in this case, the Sanofi and AstraZeneca partnership, acknowledged that despite an aggressive supply plan, demand for Beyfortus, especially the 100 mg doses for babies born before the RSV season, exceeded expectations. Orders for new doses are currently on hold.
The CDC also briefly paused ordering of the immunization through its Vaccines for Children program due to limited supply. However, they anticipate that more doses, both 50 and 100 milligrams, will become available every 2 to 3 weeks from the manufacturer.
Pediatricians and health organizations had hoped that supply wouldn’t be an issue, and the American Academy of Pediatrics has requested more information regarding the duration of the shortage. They advise against using two 50-milligram doses as a substitute for a single 100-milligram injection, as it hasn’t been studied, approved, or recommended.
In response to the scarcity, the CDC has issued a health alert advising doctors to inform pregnant individuals about the new maternal vaccine for RSV, Abryvso, which provides protection against severe RSV for newborns during their initial months of life.
Our Reader’s Queries
Why is there no RSV vaccine?
Developing effective RSV vaccines is a challenging task due to various obstacles. One of the major hurdles is the need to vaccinate very young infants who may not respond well to the vaccine. Additionally, RSV is divided into two antigenically distinct groups, A and B, which further complicates the development of a universal vaccine. Furthermore, there is a history of disease enhancement following formalin administration, which adds to the complexity of vaccine development. Overcoming these obstacles is crucial to the successful development of RSV vaccines.
Why is there an RSV shortage?
Due to an unexpected surge in demand, the medication has become scarce across the country, surpassing the manufacturer’s projections. Regrettably, CHOP and other organizations in the area will not receive any additional doses of the medication this season, as the supplier is unable to fulfill the demand.
When is the RSV vaccine available?
In the autumn of 2023, the Centers for Disease Control and Prevention and the American Academy of Pediatrics endorsed nirsevimab, an RSV vaccine. This vaccine is particularly beneficial for infants who are at the highest risk of contracting severe RSV infections. By providing protection against this dangerous virus, nirsevimab can help safeguard the health and well-being of vulnerable babies.
Is Pfizer working on the RSV vaccine?
Pfizer is dedicated to creating an RSV vaccine for adults and infants through maternal immunization, thanks to recent scientific breakthroughs. Our focus is unwavering, and we are determined to make this a reality.