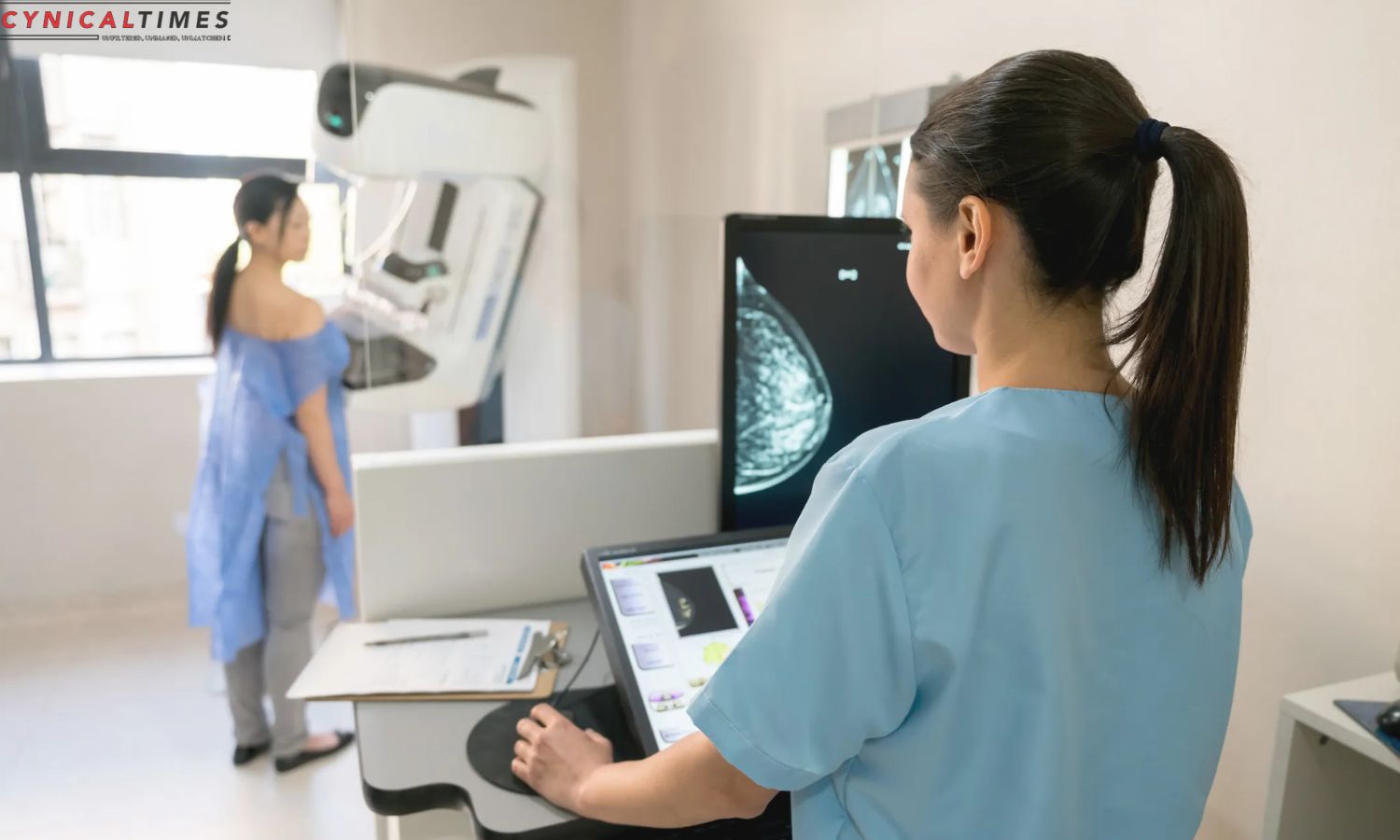
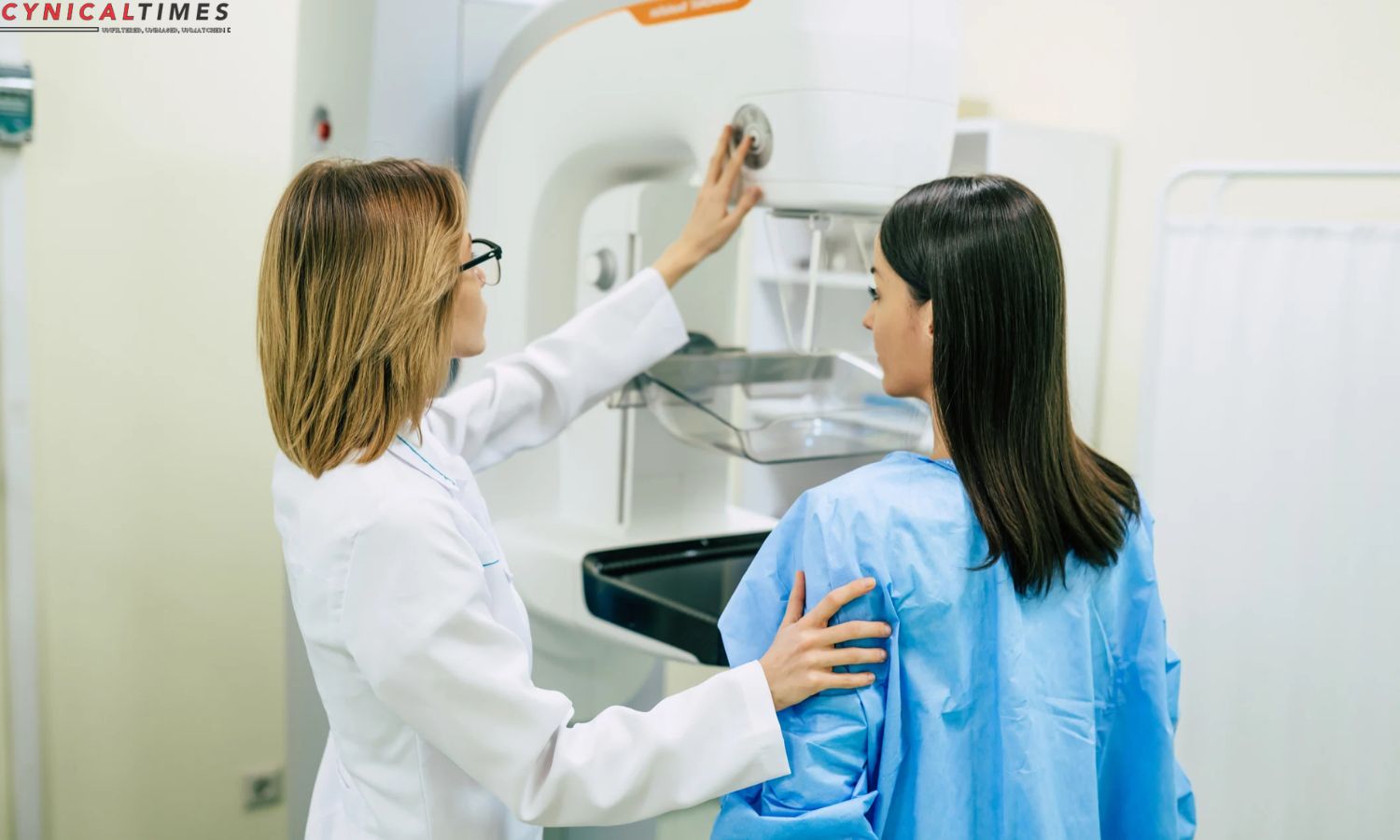
Anixa Biosciences Unveils Potential Lifesaver: Anixa Biosciences is developing a vaccine to prevent triple-negative breast cancer recurrence. Dr. Vincent Tuohy’s vaccine, developed at Cleveland Clinic, is undergoing rigorous testing and has showed promising results in early human studies.
Triple-negative breast cancer causes significant hurdles and high recurrence rates, highlighting the need for novel and effective treatments. Dr. Tuohy and his team spent over two decades developing the three-shot vaccination. Their innovative work has received FDA approval for human testing, a major step toward a better cancer treatment.
Dr. Amit Kumar, Anixa Biosciences CEO, believes the vaccine will transform cancer prevention and therapy. It fills a significant gap in treatments for aggressive triple-negative breast cancer, making the company’s emphasis important.
Also Read: Tuberculosis Scare Hits Nevada Schools: Health Officials Launch Investigation
The vaccine’s mechanism of action is centered around teaching the immune system to identify and destroy cancer cells before they form tumors. This approach represents a departure from previous attempts at cancer vaccines, which often faced challenges in achieving significant efficacy. The unique methodology employed by the vaccine offers hope for a breakthrough in preventing cancer recurrence, a critical aspect in the treatment of triple-negative breast cancer.
The ongoing human trials involve women who have been diagnosed with triple-negative breast cancer, and early indications are encouraging. Participants in the trial have exhibited immune responses, indicating that the vaccine has the potential to bolster the body’s natural defenses against cancer cells.
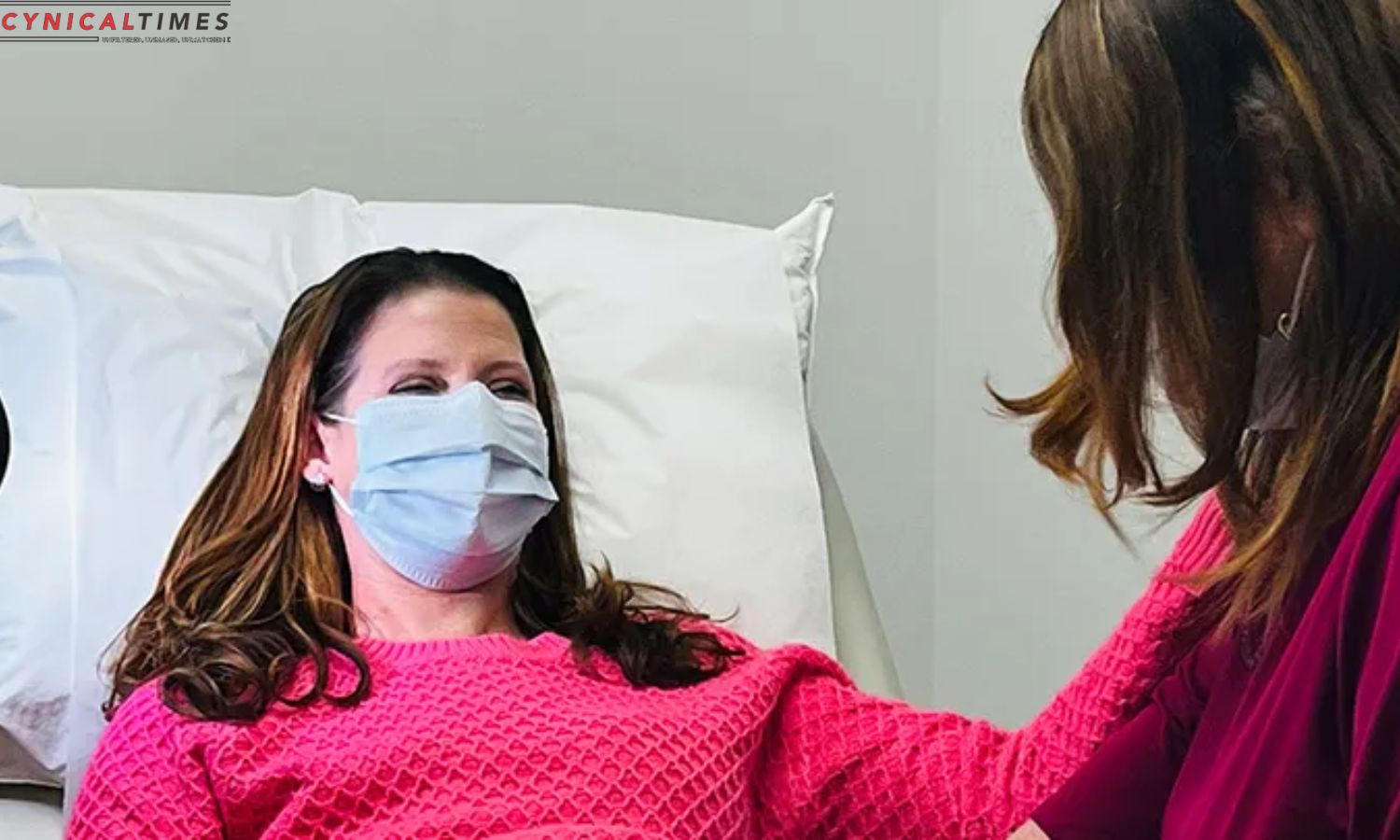
Jennifer Davis, one of the trial participants, shared her experience as the first woman to undergo the series of shots. Having faced a challenging battle with triple-negative breast cancer, Davis emphasized the importance of scientific innovation in improving treatment options. The vaccine’s success could represent a pivotal moment for individuals diagnosed with triple-negative breast cancer, offering a potential lifeline in preventing recurrence.
Anixa Biosciences plans to continue the trial, testing the vaccine on more participants as part of the phase one study. The company aims to initiate a double-blind study with hundreds of women starting in 2024. This phase of the research will provide valuable insights into the vaccine’s effectiveness in preventing recurrences compared to standard care alone.
Dr. Kumar suggested that the vaccine’s success could lead to similar vaccines for other tumors. A diverse and successful cancer vaccination would be a major advance in the fight against this dreadful illness.
Besides Anixa Biosciences, researchers at the University of Washington School of Medicine are also testing breast cancer vaccinations. These collaborations emphasize the need to advance cancer research to generate more effective and targeted treatments.
While the vaccine may not be widely available yet, Anixa Biosciences and its collaborators’ progress bodes well for a future where innovative immunotherapies prevent cancer recurrence and improve cancer patients’ lives.
Our Reader’s Queries
Is Anixa good stock to buy?
Anixa Biosciences Inc has received a Moderate Buy rating from experts, with 1 buy rating and no hold or sell ratings. The average price target for the company is $12.00, as predicted by 1 Wall Street Analyst in the past 3 months.
Who is the CEO of Anixa Biosciences?
As the current Chairman and CEO of Anixa Biosciences, Dr. Kumar is a seasoned professional who also holds positions on the Board of various public and private companies. Since 2016, he has been an esteemed member of the Board of the American Cancer Society.
Is Anixa Biosciences announces issuance of additional US patent for ovarian cancer vaccine technology?
Anixa, a biotech firm dedicated to fighting cancer, has exciting news to share. The U.S. Patent and Trademark Office (USPTO) has granted the company U.S. Patent Number 11,786,489. This patent expands the protection of Anixa’s innovative ovarian cancer vaccine technology. This is a significant development in the fight against cancer, and Anixa is proud to be at the forefront of this important work.
What is Anixa?
Anixa Biosciences Inc is a company that specializes in the development of CAR-T based immuno-therapy drugs and a COVID-19 therapeutics program that targets specific viral protein functions. Their main focus is on breast cancer and ovarian cancer therapeutics programs. With a commitment to innovation and cutting-edge research, Anixa Biosciences Inc is dedicated to improving the lives of patients through the development of groundbreaking treatments.