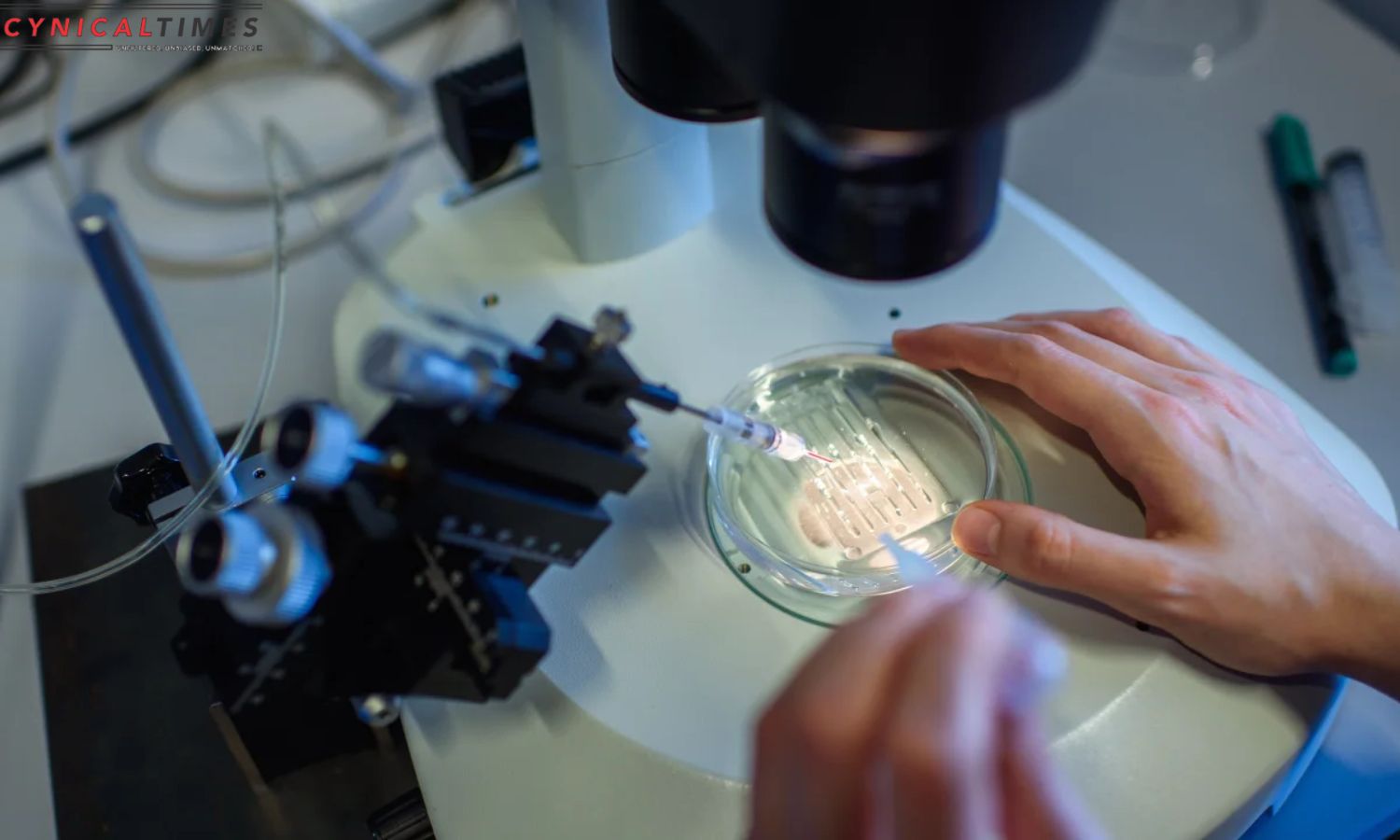
UK Makes Medical History with CRISPR: The UK became the first country to approve a CRISPR gene-editing medical treatment. The MHRA approved Casgevy, a sickle cell disease and beta thalassemia therapy. These genetic conditions, caused by hemoglobin gene errors, lack a universally successful treatment. Sickle cell disease, prevalent in individuals with African or Caribbean backgrounds, and beta thalassemia, affecting those of Mediterranean, South Asian, Southeast Asian, and Middle Eastern origin, are painful, lifelong disorders.
Previously, a risky bone marrow transplant was the only permanent treatment. However, the newly authorized Casgevy, developed by Vertex Pharmaceuticals, represents a groundbreaking gene-editing solution. Unlike traditional medications, Casgevy involves extracting stem cells from the patient’s bone marrow, editing a specific gene in a laboratory, and then reintroducing the modified cells after a conditioning treatment.
This innovative approach has shown promising results in restoring healthy hemoglobin production and alleviating symptoms in trial participants. While the UK has paved the way for this transformative treatment, the US Food and Drug Administration is currently evaluating the same therapy, with a decision expected by December 8. The Casgevy approval signifies a significant leap in medical advancements, offering a potential cure for genetic diseases that were once deemed incurable.
This breakthrough underscores the tremendous impact of CRISPR-Cas9 technology on biomedical research and clinical medicine, presenting a new era in the quest to address genetic disorders.
Despite the controversy surrounding CRISPR-Cas9, exemplified by the 2018 announcement of gene-edited babies by Chinese scientist He Jiankui, the technology’s potential to revolutionize genetic medicine cannot be denied. As the world witnesses the unfolding developments in gene editing, the approval of Casgevy in the UK marks a pivotal moment in the pursuit of innovative solutions for previously untreatable genetic conditions.
Also Read: Revolutionizing Infant Health: CDC Maternal RSV Vaccine for Early Months Safety
Our Reader’s Queries
Is the UK the first country to approve CRISPR treatment?
Yesterday, U.K. regulators made history by approving a groundbreaking therapy that utilizes the gene-editing technique CRISPR. This innovative approach is designed to treat two inherited blood disorders, including sickle cell disease, which predominantly affects individuals of African descent. The therapy involves modifying a patient’s blood stem cells in a laboratory setting and then returning them to the patient. This marks a significant milestone in the field of gene therapy and offers hope to those suffering from these debilitating conditions.
Is CRISPR used in the UK?
Utilizing CRISPR gene editing, a new therapy has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) after undergoing two successful global clinical trials. The UK arm of the trials was led by Imperial College Healthcare NHS Trust. This innovative therapy offers a promising new approach to treatment.
What is the UK Greenlights world’s first CRISPR gene editing therapy?
The UK’s regulatory body has given the green light to the world’s inaugural CRISPR-Cas9 gene editing therapy. This revolutionary treatment, known as Casgevy (exagamglogene autotemcel), is designed to cure sickle cell disease and transfusion-dependent ?-thalassemia. Developed by Vertex Pharmaceuticals and CRISPR Therapeutics in Zug, Switzerland, this pioneering therapy is a game-changer in the field of gene editing.
Which British regulators approved a sickle cell disease treatment derived from CRISPR the gene-editing therapy?
The UK regulators have given the green light to the first-ever CRISPR-based treatment, Casgevy. This revolutionary gene-editing method aims to cure sickle-cell disease and beta thalassemia, a related condition. With this approval, Casgevy has become the first-ever CRISPR-derived treatment to be approved for use in the UK. This breakthrough treatment is expected to bring hope to millions of people suffering from these debilitating conditions.